
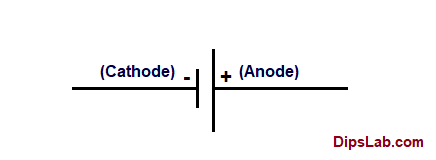
In the following examples, the anode is negative in a device that provides power, and positive in a device that consumes power: The polarity of voltage on an anode with respect to an associated cathode varies depending on the device type and on its operating mode. Therefore, this electrode is permanently named the cathode, and the electrode through which the electrons exit the tube is named the anode.Įlectric current and electrons directions for a secondary battery during discharge and charge. Similarly, in a vacuum tube only one electrode can emit electrons into the evacuated tube due to being heated by a filament, so electrons can only enter the device from the external circuit through the heated electrode. The names of the electrodes do not change in cases where reverse current flows through the device. In a diode the anode is the terminal through which current enters and the cathode is the terminal through which current leaves, when the diode is forward biased. Therefore, the electrodes are named based on the direction of this "forward" current. These devices usually allow substantial current flow in one direction but negligible current in the other direction. The definition of anode and cathode is different for electrical devices such as diodes and vacuum tubes where the electrode naming is fixed and does not depend on the actual charge flow (current). Consequently, electrons leave the device through the anode and enter the device through the cathode. Since electrons have a negative charge, the direction of electron flow is opposite to the direction of conventional current.
The currents outside the device are usually carried by electrons in a metal conductor. If the current through the electrodes reverses direction, as occurs for example in a rechargeable battery when it is being charged, the naming of the electrodes as anode and cathode is reversed.Ĭonventional current depends not only on the direction the charge carriers move, but also the carriers' electric charge. An anode is an electrode through which conventional current (positive charge) flows into the device from an external circuit, while a cathode is an electrode through which conventional current flows out of the device.

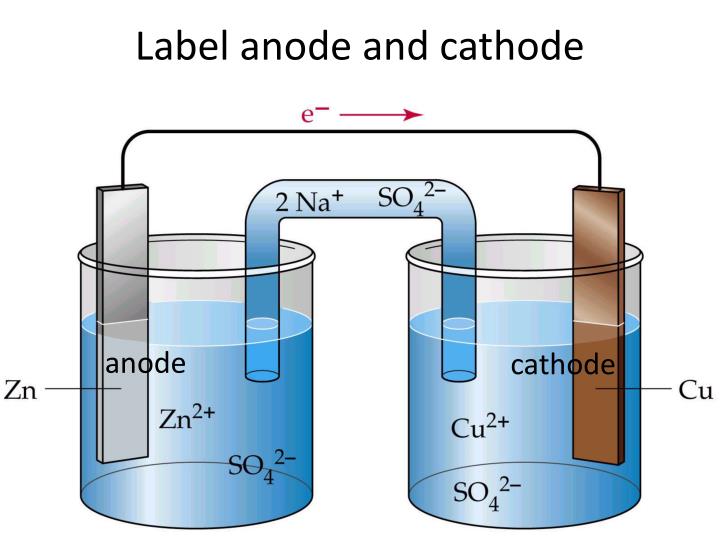
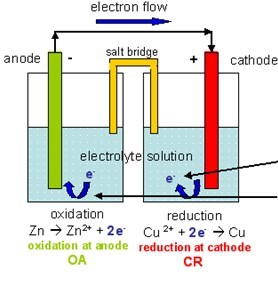
The terms anode and cathode are not defined by the voltage polarity of electrodes but the direction of current through the electrode. Historically, the anode has also been known as the zincode. Consequently, anions will tend to move towards the anode where they can undergo oxidation. In an electrolytic cell, the anode is the wire or plate having excess positive charge. In both a galvanic cell and an electrolytic cell, the anode is the electrode at which the oxidation reaction occurs. The direction of conventional current (the flow of positive charges) in a circuit is opposite to the direction of electron flow, so (negatively charged) electrons flow out the anode of a galvanic cell, into an outside or external circuit connected to the cell. A common mnemonic is ACID, for "anode current into device".
This contrasts with a cathode, an electrode through which conventional current leaves an electrical device. Note how electrons move out of the cell, and the conventional current moves into it in the opposite direction.Īn anode is an electrode through which the conventional current enters into a polarized electrical device. Diagram of a zinc anode in a galvanic cell.


 0 kommentar(er)
0 kommentar(er)
